
First Human Trial of Experimental Cancer-Killing Virus Underway
The CF33-hNIS virus (referred to as Vaxinia) is an oncolytic virus, a genetically-engineered variety that habitually targets cancer cells while ignoring healthy cells. But beyond infecting and killing cancer cells, Vaxinia works overtime by specially-engineered white blood cells, known as CAR T cells, to solid tumors. While CAR T cells are vital to helping the body’s immune system recognize cancer cells as a threat, solid tumors possess immunosuppressive microenvironments that act as barriers, preventing the CAR T cells from entering and doing their job.
“Our City of Hope team designed this CF33 oncolytic virus to do what it does so well. It enters the cancer cell, uses the cell’s own machinery to replicate itself, and engineers the cancer cells to express the well-known CAR T cell target, CD19,” City of Hope’s professor of surgical oncology Dr. Yuman Fong said in a .
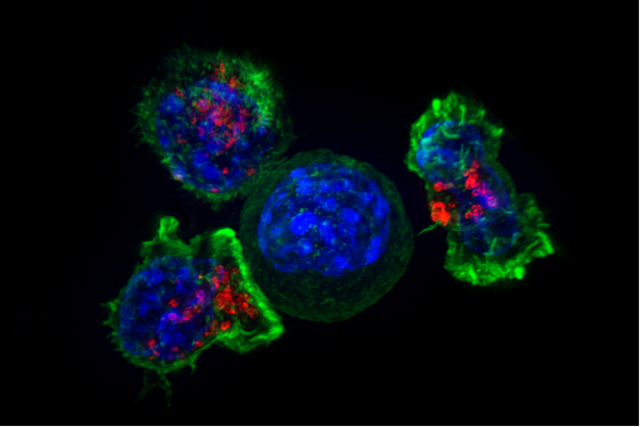
A group of T cells (green and red) surrounding a cancer cell (blue). (Photo: The National Institutes of Health/Wikimedia Commons)
City of Hope has been working with biotechnology company Imugene Limited to develop Vaxinia since late 2020. While the two organizations have tested Vaxinia in mice, they haven’t witnessed how the virus performs in the human body—that is, until now. The first human trial began last month and will last through the end of 2024.
A total of 100 participants will receive treatment through the Phase I . Though all of them will receive Vaxinia on the first and eighth days of the first portion of the trial, some will receive it via IV, while the rest will receive direct tumor injections. Additionally, some participants will undergo what’s referred to as “combined” treatment using a drug called pembrolizumab, which has historically been used to treat specific types of cancers.
The trial will help calibrate the ideal dosage for adult patients and determine whether Vaxinia is safe for human use. If the trial is successful, Vaxinia could be brought to larger participant groups—and even everyday healthcare settings—in the future.
Now Read: